Immunotherapy of Solid Tumor Cancers: Role of Cancer Associated Fibroblasts
December 12, 2017
Cancer therapy traditionally involves surgery, radiation or chemotherapy to kill cancer cells that are normal cells lacking control that limit growth. Cancer cells produce continually growing tumors that spread to other tissues, and eventually kill. Since the early 1990’s, other targeted therapies have been developed with fewer side-effects. During 2017, the first CAR-T immunotherapies were FDA approved for treatment of both leukemia and lymphoma.
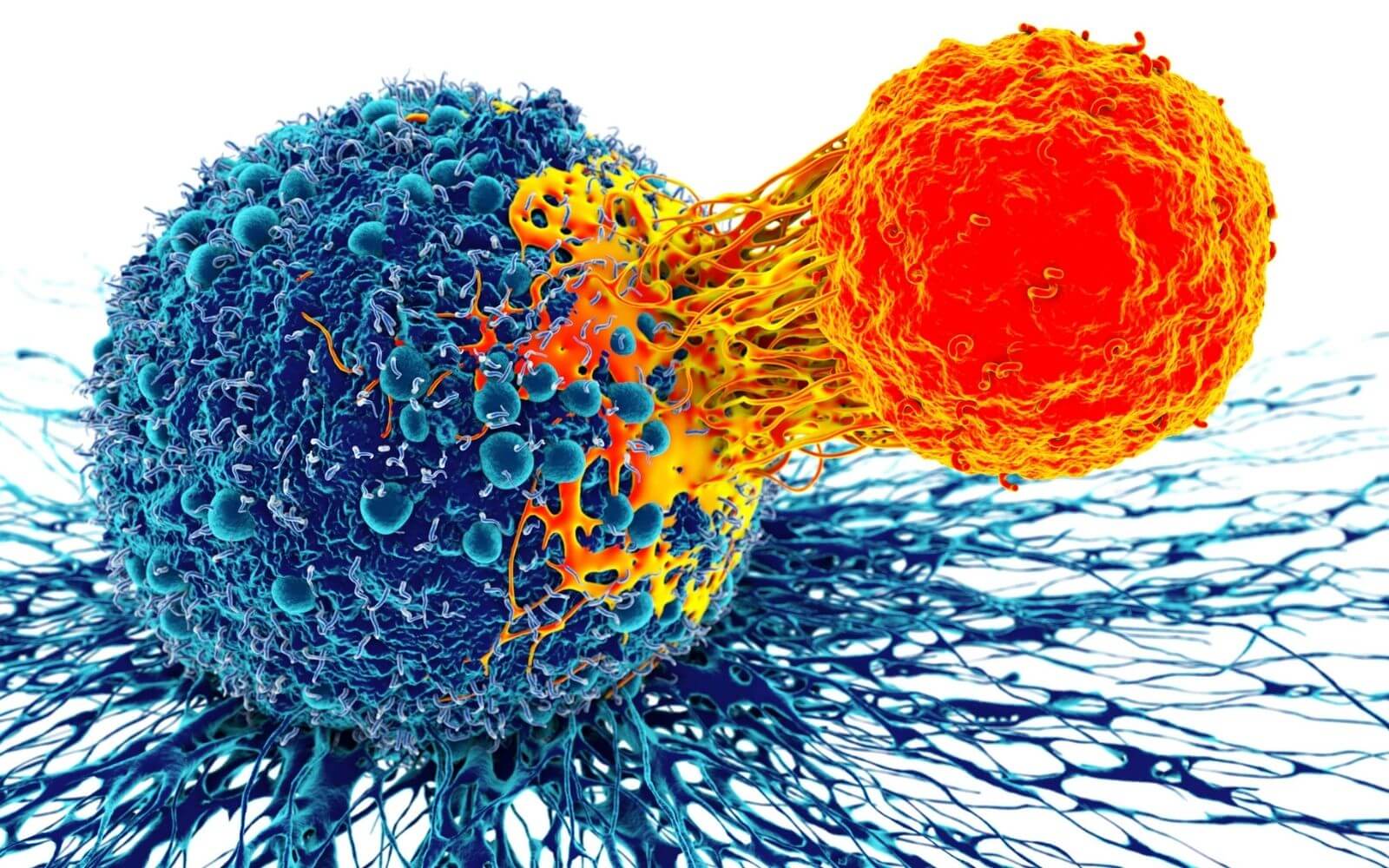
CAR T-cell therapy involves genetic engineering of patient’s T cells (an immune cell that attacks foreign cells) so they attack and destroy cancer cells. T-cells are taken from patient’s blood, expanded, and the gene for the cancer’s antigen is expressed in the T-cells. The special antigen is called a chimeric antigen receptor (CAR). Large numbers of the CAR T-cells are grown in the laboratory and infused back into the patient[1].
CAR T-cells bind to antigens present on the cancer cell surface, and then selectively destroy just the cancer cells resulting in minimal side-effects. This works very well with blood cancers such as leukemia and lymphoma. However, CAR T-cell therapy has reduced effects on solid tumors found in breast, lung, pancreatic, and other solid tumors. This is because tumor antigens reside on the surface of cancer cells buried within the tumor, where they are surrounded and protected by cancer associated fibroblasts (CAFs) [2].
Cancer associated fibroblasts are a subpopulation of cells that reside within the tumor microenvironment that promote the transformation process by encouraging tumor growth, angiogenesis, inflammation, and metastasis[3]. These cells have been shown to provide a barrier to solid tumors by acting as a defensive shield, blocking the interaction of immune cells and CAR T-cells with cancerous cells.
Vitro Biopharma manufactures CAFs and media to support CAF expansion. We offer CAFs from endometrial adenocarcinoma, colorectal, lung adenocarcinoma, lung squamous carcinoma, pancreatic, breast, melanoma, and ovarian tumors. All CAFs are fully characterized by growth rates, maximum passage number, and expression of the biomarkers: alpha-SMA, FAP, GFAP, FSP1, CD44, CD90, CD105, CD126, CD133, CD326 by flow cytometry and/or IHC. We have sold these products to many different customers in academia and the biopharmaceutical industry for several years. We offer extensive technical support of these products, opportunities for partnership/custom CAFs, multi-cell cultures and 2 or 3-D configuration. Vitro Biopharma is a biopharmaceutical manufacturing entity with strict adherence to FDA-GMP guidelines. For additional information see: www.vitrobiopharma.com
By: Tiana Tonrey, MS, Laboratory Manager, Vitro Biopharma, Richard Dean, Research Associate, Vitro Biopharma and Krutika Patell, Laboratory Technician, Vitro Biopharma
References:
- NCI Dictionary of Cancer Terms. NIH; [accessed at 2017 Dec 11]. https://www.cancer.gov/publications/dictionaries/cancer-terms?cdrid=771302
- CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. NIH; [updated 2017 Aug 31; accessed at 2017 Dec 11]. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- Madar, S. Goldstein, I. Rotter V. Aug 2013. ‘Cancer associated fibroblasts’ – more than meets the eye. Trends in Molecular Medicine. [accessed at 2017 Dec 11];19(8):447-453.
Also in Blogs
Stem Cell Therapy of Stroke: Current Research Support and Clinical Studies

Anti-Aging Stem Cell Therapy Improves Quality of Life